The overarching objective of imSAVAR is to enhance the capacity for predicting adverse immune-related toxicities and establish a standardized approach to immune safety assessment.
To achieve this, we aim to refine existing models and develop novel, fit-for-purpose models that accurately represent the complexity of the human immune system. By doing so, we strive to improve our ability to effectively treat, prevent, and even predict immune-related adverse events.
In addition, we will establish a comprehensive platform that serves as a guiding framework for the development of nonclinical safety assessment strategies specifically tailored to immunotherapeutics and their mode of action within the human system. Even beyond the project’s funding period, this platform will be sustained through the collaborative efforts of a dedicated stakeholder community, which will continue to innovate new assessment strategies and support the implementation of existing ones.
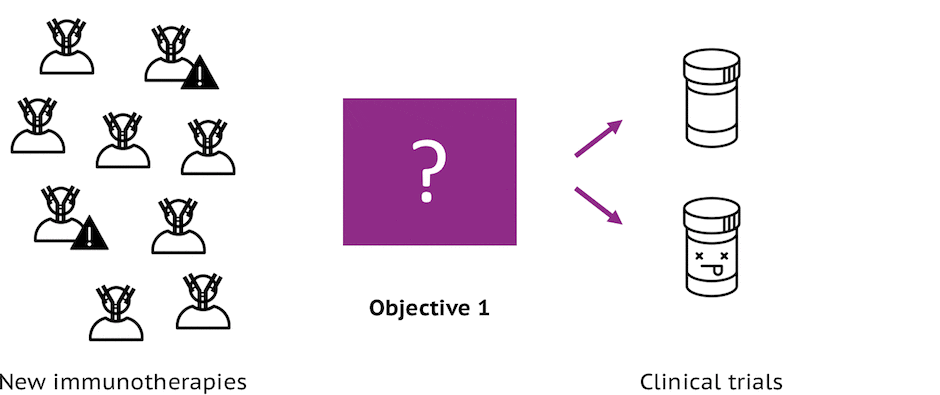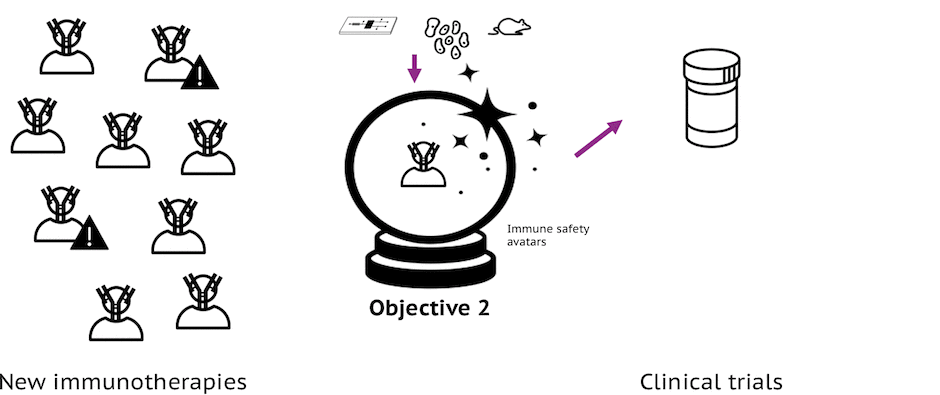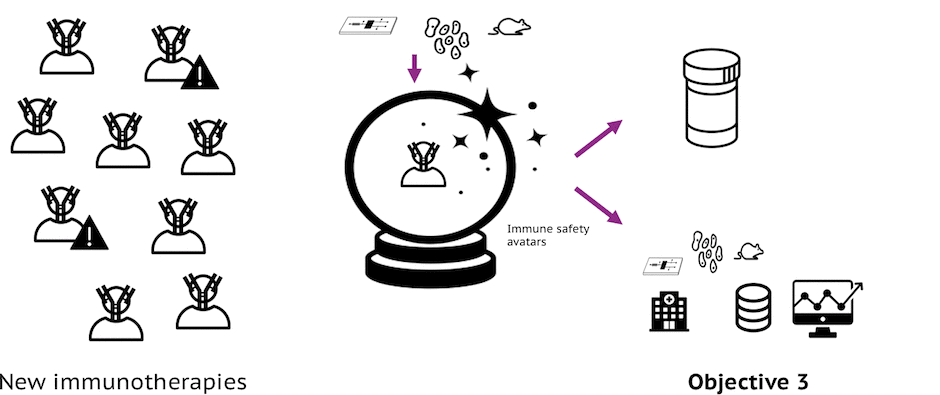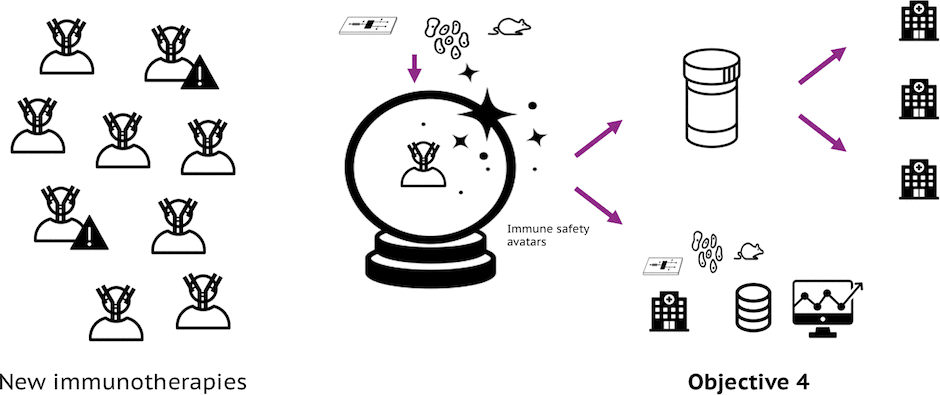
Objective 1: ID MoAs
Identification of immunomodulatory modes of action (MoAs) that have insufficient methods for predicting human toxicities so that resources can be focused where they are most likely to lead to real differences in predicting immunosafety.
Objective 2: Refine & Build Models
Refine existing models, develop new models, and identify biomarkers for preclinical assessment of efficacy and safety for immuno-oncology and immuno-inflammatory therapeutics so that development of unsafe immunomodulatory therapies can be stopped early in the development process.
Objective 3: Create a Platform
Creation of a platform that combines models with a network of biosample resource providers that can carry out immune safety assessment strategies in an efficient manner and continually to progress the field.
Objective 4: Outreach beyond Funding Period
Creation of an immune safety stakeholder community and business models that can maintain and extend the vision of imSAVAR beyond the funding period.